Making Hydrogen Affordable
Green hydrogen could be critical for chemical production, but making it cost effective is a tall task.
How Electrolyzers Work
Basics
Atoms make up the world, and electrons are one part of an atom. How atoms share electrons determines how they bond and what molecules they form. Electrolyzers apply force (voltage) to change how atoms share electrons to create different compounds. In practice, one reaction is donating electrons (oxidation), and another is accepting them (reduction). The donation happens at the anode and acceptance at the cathode.
Splitting water to make hydrogen and oxygen is the classic example. The half-reactions in a proton exchange membrane (PEM) style machine are:
(1) H2O -> O2 + 2H+ + 2e at the anode and
(2) 2H+ + 2e -> H2 at the cathode.
In every electrochemical cell, an ion (H+ in this case) has to cross the cell by traveling through the electrolyte. The membrane is the electrolyte in PEM electrolyzers since ions struggle to travel through pure water. The electrons travel through a wire from the anode to the cathode.
Another major style is alkaline water electrolysis. The half-reactions are:
(1) 2OH- -> 0.5O2 + H2O + 2e
(2) 2H2O + 2e -> H2 + 2OH-
A caustic salt like potassium hydroxide is the typical electrolyte.
Other products commonly produced by electrolysis are aluminum, magnesium, sodium/potassium hydroxide, chlorine, and sodium metal.
Measuring Efficiency
Every pair of electrochemical reactions has a minimum amount of force, measured in voltage, to make it go. Many sources of resistance add to how much voltage it takes to run the reaction in the real world. The theoretical voltage divided by the total voltage is the efficiency. The minimum for splitting water is 1.23 volts, so an electrolyzer running at 2 volts would be ~62% efficient.
The primary sources of resistance are:
Anode/Cathode Catalyst Overpotential
There are no perfect catalysts, and they require extra voltage to run the reaction at a reasonable rate.
Electrolyte Resistance
Ions need force to push them between the electrodes, and the required force increases as current (electron flow) and distance increase. Gas bubbles also make it more difficult for ions to move through the electrolyte.
Separator Resistance
Ions can struggle to get through the membranes/diaphragms/separators that separate the anode and the cathode.
These factors vary based on the current. Resistance goes up as current does, just like an electrical wire. Increasing the current increases the reaction rate (and device productivity), but efficiency suffers, creating a classic tradeoff.
Today's Electrolyzers
The resistance in the electrolyte forces each cell to be very thin. Engineers typically increase the area of a cell until it becomes challenging to maintain a uniform current across the catalyst surfaces, which is one square meter as a rule of thumb. Scaling these systems is not nearly as graceful as a thermochemical reactor because it involves stacking hundreds or thousands of cells instead of making a vessel bigger. Putting the cells in "stacks" helps to save on piping, wiring, and materials costs.
Alkaline and PEM stacks. Source
Alkaline electrolyzers have been the historical leader and produced hydrogen for 30% of global ammonia production around 1930. PEM electrolyzer market share has grown because of their higher efficiency and smaller footprint. Other niche types, like solid oxide electrolysis, can be more challenging to pair with clean, variable electricity because they are expensive and operate at high temperatures. The market for hydrogen from water splitting is tiny compared to the production of hydrogen from coal, oil, and natural gas in 2023.
The Economics of Electrolysis
Successful electrochemical production processes seem to have three traits:
High Selectivity
The lack of side products allows increases in voltage and current density (productivity per unit of area) without faradaic efficiency (electrons doing what you want instead of something else) falling and compromising any production gains.
Product is Saleable
Any post-processing like distillation is a killer given the higher cost and complexity electrochemical processes usually have. Products need to separate from the electrolyte with high purity.
High Value Per Electron
The value of the product each electron creates is a proxy for operating costs and capital costs.
Water electrolysis using reasonable catalysts and electrolytes has good selectivity. The threat to product purity is hydrogen and oxygen mixing, requiring an expensive membrane separator or careful design. The primary issue is the value per electron. Hall-Heroult (aluminum), Chlor-Alkali (sodium hydroxide and chlorine), and the Rowley Magnesium process produce about 20x the value per electron as classic water electrolysis selling hydrogen for $1/kg (a price relevant for chemical feedstocks). Hydrogen isn't worth that much and each electron produces very little saleable mass because 7/8's of water's mass is near-worthless oxygen.
Why Producing Oxygen is Still Worth It
A feature of electrolysis is that it always produces two products, one reduced and one oxidized. One idea is to make a different product than oxygen at the anode. But the products we want to use hydrogen-based processes to replace are some of the most used substances on earth. Even with drastic reductions from electrification, we might still be using billions of tons. There are no co-products for hydrogen evolution that have markets large enough. The next best thing is a co-product with zero disposal cost. Oxygen evolution fits the bill.
Alternative ways of getting the hydrogen into fuels and chemicals don't work well. CO2 electrolysis is complex and almost always paired with oxygen evolution, anyway. Biomass is expensive and difficult to scale, besides often having a large footprint.
Scaling synthetic chemical and fuel production almost certainly requires living with the waste of oxygen. It would be a significant advancement if anyone did develop a co-product that could sell in the billions of tons.
What Cheap Hydrogen Can Do
Hydrogen is a terrible fuel because of its low energy density, propensity to leak, and tendency to embrittle metals. But, it is a popular chemical feedstock, forming a fundamental building block for many chemicals. Hydrogen and carbon dioxide can form methane and methanol in easily scalable processes. Hydrogen and CO2 can be processed into syngas (carbon monoxide and hydrogen) using long-industrialized water-gas shift reactions. Syngas can make a range of liquid hydrocarbons, like jet fuel. These are relatively simple processes, often with long industrial histories, that aren't dominant because getting crude oil out of the ground is cheaper than making hydrogen from hydrocarbon feedstocks and producing fuel in a large facility. Hydrogen must be much less expensive than fossil fuel-derived hydrogen to beat crude oil and other fossil hydrocarbons.
Hydrogen at $1/kg can enable the production of ammonia or methanol and its derivatives at competitive rates but will still be too expensive for methane or jet fuel. $0.50-$0.75/kg is a more reasonable goal for transformative change.
The Cost of Production
Electricity
The embodied energy in hydrogen is ~39 kWh/kg. The electricity required to produce the hydrogen is this number divided by the efficiency. An 85% efficient electrolyzer would require ~46 kWh/kg of hydrogen. Industrial electricity prices range from $0.05-$0.10/kWh in most industrialized countries, implying an energy cost of $2-$4/kg of hydrogen.
Those prices are devastating for a $0.50-$1/kg goal. Even a 100% efficient electrolyzer won't work. Electricity must be around $0.01/kWh with realistic efficiency assumptions. Co-location of electricity generation is the only choice because transmission and distribution costs exceed $0.02/kWh. Anything that can produce for under $0.01/kWh works, but solar PV has several advantages:
Solar panels produce the same type of electricity that electrolyzers use, low voltage direct current(DC), reducing conversion losses/costs.
Solar PV DC costs only ~70% of Solar PV AC electricity.
There is a viable path to reducing DC costs to $0.01-$0.015/kWh through better efficiency and solar farm designs.
Solar looks promising, but it is only available for part of the day! Storing electricity will be too expensive, so any electrolyzers hooked directly to solar panels will suffer lower capacity factors and higher capital costs. The electrolyzers must be next-level cheap to build.
Feedstock
The primary feedstock is water. Water is usually obscenely cheap, but most water electrolyzers require very pure deionized water, which can add ~$0.05-0.10/kg of hydrogen (especially for PEM electrolyzers). The obvious thing is to have a process that tolerates city water or only requires minimal extra treatment. The deionized water in fancy electrolyzers is necessary to prevent fouling of the membranes and catalysts, which are incredibly expensive. Removing or substituting those items will reduce capital and feedstock costs.
Labor and Maintenance
Estimates are as high as $0.30-$0.40/kg for operations and maintenance costs for green hydrogen production.
Operations labor isn't typically a significant component of production costs, but scale matters. Small deployments often need at least one operator, but they aren't busy. Scaling up can allow the labor to go further.
Maintenance can be more substantial due to maintaining water purification systems, compressors, and replacing catalysts/membranes. The easiest way to avoid these costs is to have fewer of these systems, which aligns with using less pure water, lowering capital expenditure, and having local low-pressure hydrogen users.
Capital Expenditure
Reducing complexity and capital expenditure is the lynchpin in delivering sub-$1/kg hydrogen. The primary tradeoff available is decreasing efficiency and productivity per square meter of electrode in exchange for cheaper materials. The cost needs to fall to ~$30 per kilowatt! Current designs have many costly features:
High Pressure Systems
Producing hydrogen in a small area creates bubbles that interfere with ions traveling through the electrolyte. Ramping up pressure makes these bubbles smaller. There is also an assumption that hydrogen must be at high pressure, and high-pressure electrolyzers can reduce compression costs. PEM electrolyzers require expensive materials like stainless steel or titanium to contain the pressure without corrosion, while alkaline electrolyzers use steel.
Membranes
Losses from any resistance in the system grow as the current increases. Electrode gaps must be small in high-productivity devices (zero gaps are best!). Membranes prevent electrodes from touching and causing short circuits. They also limit the crossover of hydrogen gas while facilitating the transport of ions between electrodes. Crossover is much worse when pressure increases, requiring more expensive materials to minimize gas transport while maximizing ion transport. PEMs use their namesake proton exchange membranes and typical Alkaline designs have less expensive diaphragms but use more total area. These membranes wear out and are vulnerable to fouling, requiring pure water.
Catalysts
High-productivity electrolyzers need the best catalysts, like platinum or iridium, to limit overpotential.
Pure Water Production and Recycling
Not only does deionized water cost a lot to produce, but it drives other process complexity. Excess water is cooled and degassed before returning, requiring extensive equipment and piping.
Fragile Architecture
Almost every electrolyzer is in a structure. The cost of these structures is another driver favoring PEMs because they can be 20x smaller in volume than traditional alkaline electrolyzers. The building alone can blow the budget.
Any low-cost electrolyzer must ditch titanium/steel for plastic or another inexpensive material, operate without a membrane, and utilize more affordable catalysts while maintaining product purity and safety. It must work at lower capacity factors to take advantage of cheap electricity. The process looks like making alkaline electrolyzers cheaper. The design features are:
Atmospheric Pressure
Pressure very quickly increases the design complexity and cost of a vessel. An atmospheric electrolyzer can be plastic. Gas purity increases because less gas can dissolve in the fluid and crossover.
Alkaline Electrolyte
Catalysts compatible with alkaline electrolytes are a fraction of the cost of those effective in acidic electrolytes. Corrosion of components is not as dramatic, and ionic conductivity is acceptable. Hot, alkaline electrolytes carry significantly less gas than pure water.
Reasonable Electrode Gaps
A system operating at reasonable current densities can have enough room to eliminate the membrane, manage bubbles, limit product crossover, avoid circulating the electrolyte, and not suffer too much resistance.
Fed Directly with DC
Energy conversion is expensive in equipment and efficiency. Solar panels produce low-voltage DC, and electrolyzers consume low-voltage DC. Any conversions are a waste. Both the solar panels and electrolyzers can be organized in series to produce and consume voltages that are reasonable for wiring.
Control systems can also bust the budget. An atmospheric pressure electrolyzer without a membrane/diaphragm should have a wide operating range. Engineers should pursue every effort to hook banks of panels up directly to banks of electrolyzers, avoiding power electronics and controls.
Live Outside
Buildings are expensive! The design must be robust enough to be outside (like typical chemical plants).
Membraneless
Removing the membrane reduces costs, allows for low-quality feed water, and eliminates the risk of pressure gradients between compartments that cause gas crossover.
Even with these simple designs, there are still avenues for improvement. Catalysts can improve to have lower overpotential at higher currents. Expensive material can be applied thinner and thinner on cheaper substrates. Clever plastic "bubble guides" might maintain product purity with smaller electrode gaps and higher current density. The details can become incredibly complex even with a "simple" architecture.
Variable Operation
Alkaline electrolyzers often perform poorly with regular cycling up and down. The primary issues are power electronics and hydrogen crossover into the oxygen stream, creating an explosive mixture. Further inspection shows that these issues stem from complexity and mostly disappear in a simple electrolyzer.
Machines connected directly to panels don’t have power electronics that hold them back or operate inefficiently at low loads. They cannot utilize every bit of energy the panels produce when directly coupled, but losses can be lower than using power electronics to optimize voltage and current. Deleting the parts reduces cost and maintenance.
Hydrogen crossover tends to be constant, while oxygen production varies with the power input. At low loads, the ratio of hydrogen in the oxygen stream can be dangerous. The crossover is worst in zero-gap configurations and at higher pressure. An atmospheric pressure machine with reasonable electrode gaps can have dramatically lower minimum safe loads. Another failure method is pressure differences between the cathode and anode compartments increasing crossover when using a diaphragm/separator. A machine without a diaphragm is much easier to keep equalized.
The upshot is a simple alkaline electrolyzer is nearly as tunable as PEM electrolyzers.
Post-Processing
The cost targets for hydrogen are so low that something as mundane as compressing the product gas can blow out the entire budget. The conditions for downstream hydrogen usage are as critical as its production.
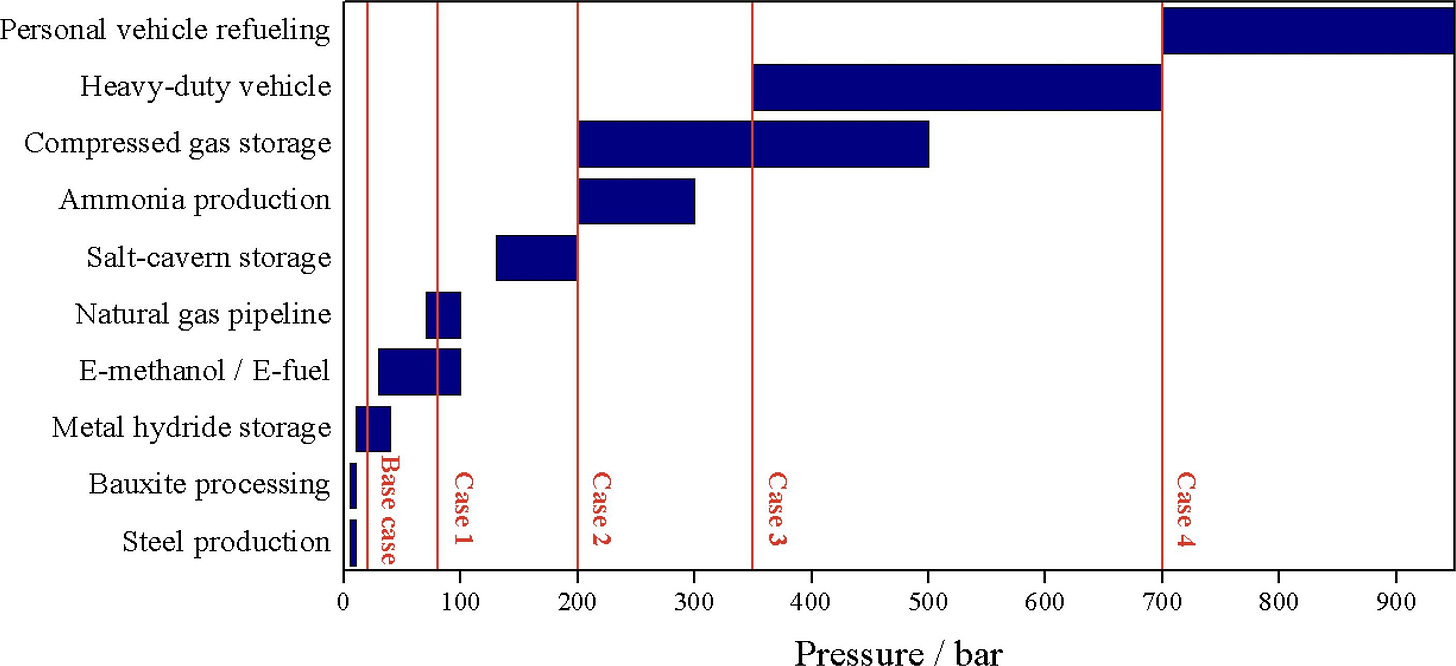
Attacking the Pressure Question
One of the main justifications for high-pressure electrolyzers is that many applications use high-pressure hydrogen, and pressurizing the electrolyzer can eliminate the compressor. But closer inspection reveals that the high-pressure use cases tend to be the silly stuff like hydrogen cars, while the more practical applications like chemical feedstocks are lower pressure.
From an advocate of high pressure electrolyzers Source
Many of the chemical processes could accept even lower pressure. The current paradigm is to turn coal or natural gas into syngas (a mixture of hydrogen and carbon monoxide) to produce methanol or other products. Natural gas enters the facilities at 50-80 bar, and coal gasification operates more efficiently at 10-40 bar. There is no reason to lower the pressure to feed downstream processes because that would require bigger vessels (gas volume increases as pressure decreases). The math is often different when starting from atmospheric pressure, especially because hydrogen is expensive to compress.
Pressure is beneficial when producing methane, methanol, and other synthetic fuels from carbon dioxide, carbon monoxide, and hydrogen. In each case, there are fewer product molecules than reactant molecules. High pressure pushes the reaction towards the products that take up less space. But there are other ways to push the reaction towards products. Removing the products (water, methanol, etc.) from the stream and recycling the leftover reactants or sending them to a second-stage reactor accomplishes the same task as pressure. Half the nitrogen in your body went through a similar recycling process in a Haber-Bosch ammonia plant!
Sub 20 bar pressures also allow a switch from expensive items like stainless steel piping to plastic piping made of high-density polyethylene (widely used in the oilfield).
We can eliminate the requirement for high-pressure hydrogen once we reevaluate the entire problem space and benefit from savings in cost and complexity.
Ensuring Quality
The primary hydrogen quality concerns come from oxygen and water.
Oxygen is relatively easy to deal with if the downstream process can't tolerate oxygen. Catalysts will facilitate a reaction with the oxygen and hydrogen in the stream, producing water. Many processes can handle a small amount of oxygen, though.
Water is much more expensive to eliminate but is only relevant for silly hydrogen applications like cars or pipelines. It shouldn't be a concern for chemical and fuel production.
A third issue could be the entrainment of the electrolyte, which may require a simple demister (like a big bunch of steel wool) if it is an issue.
Execution in Manufacturing and Design
Building these things at scale to be inexpensive, reliable, and meet specifications is hard! There are dozens of design choices with endless tradeoffs even within this simplified framework. Terraform Industries is one example of a company headed down this path. There are probably others I don't know about. The highest probability of success comes when competitors pursue slightly different iterations and push each other.
Some choices seem non-negotiable: direct coupling to delete power electronics, avoiding buildings, eliminating almost all post-production purification, and moderate pressures. There is wiggle room within the stack. If a design could affordably increase productivity while maintaining flexibility and purity, it might be more cost-effective than a stack with cheaper materials. The most aggressive cost decline estimates for traditional designs assume most reduction will come from increasing productivity. The calculations suggest costs will still be 2x-3x too high before considering the higher balance of system costs their choices impose. More creative ideas are necessary, like using a plastic membrane to wick the electrolyte to the electrodes to increase current density and efficiency without bubble interference, pressure, or increasing hydrogen crossover risk. Eventually, paths will have to converge on simple designs.
The Sad Case of Subsidies
The hydrogen and fuel cell industries are rich with firms better at obtaining subsidies than generating cash. The massive hydrogen subsidies in the Inflation Reduction Act exacerbate the issue. The subsidy pricing allows opportunists to purchase hideously expensive off-the-shelf PEM electrolyzers and do silly things with hydrogen, like compress it up to hundreds of bar, transport it in stainless pipes, etc.
Many will talk about learning curves and other cost reduction measures, and the learning curve for technologies like solar PV has been great. But Solyndra, CIGS startups, concentrated solar PV, and solar thermal did not contribute to the learning outside of inflicting pain on anyone pursuing them! Those branches were dead ends full of dead money. Massive scaling of polysilicon production and incremental improvements of the simplest technology won the day. Electrolyzer technology pathways that don't consider major simplification are dead ends because many of their components are inherently expensive or already mature, making them less prone to learning curve miracles. The hope seems to be that the industry can carve out something like corn ethanol subsidies if costs are only a few times more expensive than fossil alternatives, which is depressing.
An alternative subsidy regime might involve the Department of Energy buying a set dollar amount of synthetic fuel/chemicals for government users and allowing companies to bid for supply contracts. A commitment like this enables scaling at a reasonable price while fostering competition.
The faster the government rationalizes subsidies, the less money, human effort, and time we will waste on impractical stuff.
Conclusion
There is a path to affordable hydrogen, but it is treacherous.
Continuing the solar strategy is one of the few pathways that might deliver an affordable product without permanent government assistance. Scale polysilicon -> incrementally improve basic panel technology -> use the cheaper electricity to power the simplest electrolyzers and incrementally improve the designs -> optimize downstream processes for low-pressure, crude hydrogen.
The margin for success is narrow enough that there is no room for extra complexity or process - it is a game of pennies. Only vicious market competition can deliver that! The current US policies only delay the inevitable shakeout.